Background:
It is estimated that one-quarter to one-half of people diagnosed with hematologic malignancies experience anemia and red blood cell (RBC) transfusion plays an essential supportive role in their management. There are different strategies for RBC transfusions to treat anemia. A restrictive transfusion strategy seeks to maintain a lower hemoglobin level (usually between 70 g/L to 90 g/L with a trigger for transfusion when the hemoglobin drops below 70 g/L), whereas a liberal transfusion strategy aims to maintain a higher hemoglobin (usually between 100 g/L to 120 g/L, with a threshold for transfusion when hemoglobin drops below 100 g/L). The most effective and safest strategy is unknown in people with hematological malignancies.
Objectives:
To determine the efficacy and safety of a restrictive versus liberal RBC transfusion strategies for people diagnosed with hematological malignancies treated with intensive chemotherapy, radiotherapy, or both, with or without a hematopoietic stem cell transplant (HSCT).
Methods:
Review was completed using Cochrane standard methodological procedures. We included randomized controlled trials RCTs and prospective non-randomized studies NRS that evaluated a liberal compared with a restrictive RBC transfusion strategy in children or adults with malignant hematological disorders receiving intensive chemotherapy/radiotherapy or undergoing HSCT. We searched for RCTs and NRS in MEDLINE, Embase, CINAHL, Cochrane Central Register of Controlled Trials, and eight other databases and three trial registries to 21 March 2023. For dichotomous outcomes such as mortality, results were presented as a risk ratio (RR) with 95% confidence interval (CI) to compare restrictive versus liberal transfusion strategies. For continuous outcomes using the same scale, we assessed the mean difference (MD) with 95% CI. Where outcome measures were heterogenous, a narrative synthesis was used. Review Manager Web was used to meta-analyze the data. GRADE rating methodology was used for the overall certainty of evidence from included studies.
Main Results:
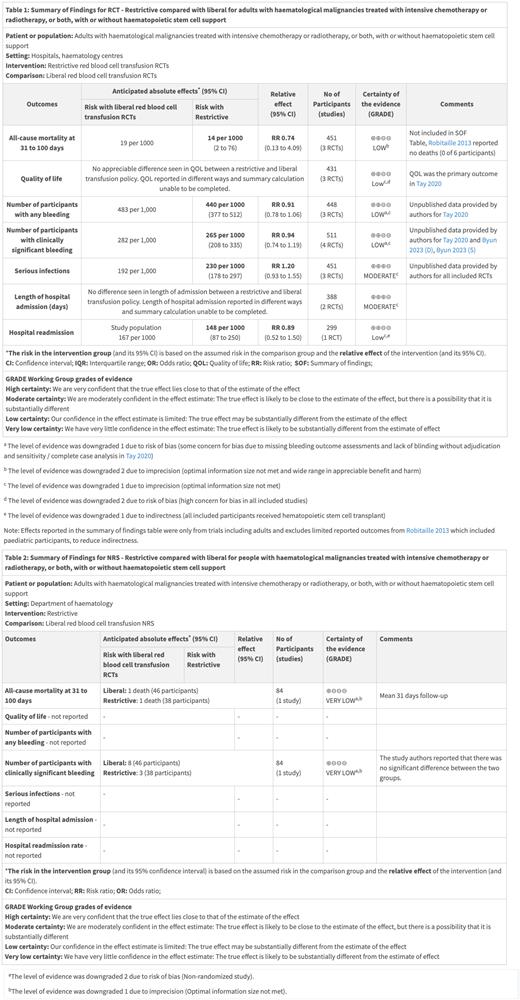
Nine studies were included: eight RCTs and one NRS. 644 participants were included in analyses from six completed RCTs (n=560) and one completed NRS (n=84). Two ongoing RCTs consisting of 294 participants (260 adult and 34 pediatric) are pending inclusion. One completed RCT included children receiving HSCT (n=6) and five RCTs only included adults (n=239 with acute leukemia receiving chemotherapy and n=315 with a hematological malignancy receiving HSCT). The restrictive strategies varied from 70 g/L to 80 g/L and liberal strategies from 80 g/L to 120 g/L. A restrictive strategy was found to result in a reduction in the number of RBC units per participant compared to a liberal strategy (Four studies; 457 participants; MD: -2.39, 95%CI -3.38 to -1.39, P = <0.00001). No difference was seen in bleeding between strategies although there was high concern for bias in included trials. Effects were reported in the summary of findings tables for RCTs (Table 1) which included results from adults only to reduce indirectness as a result of limited information from the single published pediatric RCT which was terminated early. Overall, a restrictive RBC transfusion strategy may result in little to no difference in the risk of death at 31 to 100 days compared to a liberal transfusion strategy (three studies; 382 participants; RR 0.74, 95% CI 0.13 to 4.09, P= 0.73; I 2=28%; low-certainty evidence). Two outcomes (mortality at 31 to 100 days and clinically significant bleeding) were reported from the one NRS (Table 2). The evidence is very uncertain whether there is a difference in mortality and clinically significant bleeding between the transfusion strategies based on the very low-certainty evidence.
Conclusions:
A restrictive transfusion strategy demonstrated a reduction in RBC usage, while there may be no impact on mortality. Definite conclusions are challenging given the relatively few studies with low numbers of included participants, concern for bias, heterogeneity of intervention and outcome reporting and overall certainty of evidence. There is a need for larger and methodologically better designed and executed studies. There is insufficient evidence to answer this review's primary outcome. Further RCTs are required overall, particularly trials inclusive of pediatric participants.
Disclosures
Arnold:Amgen: Consultancy; Medison: Consultancy; Canadian Institutes of Health Research: Research Funding; Argenx: Consultancy; Novartis: Research Funding; Rigel: Research Funding; Novartis: Consultancy; Rigel: Consultancy; Sobi: Consultancy; Alpine Immune Sciences: Consultancy; Sanofi: Consultancy; UpToDate: Patents & Royalties: Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal